
Hypertrophic signaling pathways are initiated through mechanical stimulation such as pressure overload and neurohumoral mechanisms including the release of signaling factors such as growth factors, hormones, cytokines, and chemokines ( Heineke and Molkentin, 2006). Many pathways have been identified to be involved in the development of cardiac hypertrophy including calcineurin/nuclear factor of activated T cells (NFAT), mitogen-activated protein kinase ERK, small guanosine triphosphate (GTP)-binding proteins (Ras, Rho), PKC, transcriptional regulation, cell surface level control, miRNA, and many more ( Stansfield et al., 2014). The main focus in hypertrophy research lies in the investigation of signaling pathways, gene expression analysis, and production of certain proteins and transcription factors, which influence or are responsible for the remodeling process. Pathological hypertrophy leads to the loss of functional cardiomyocytes ( Nakamura and Sadoshima, 2018) and can subsequently progress to heart failure with reduced ejection fraction (HFrEF) ( Tham et al., 2015). Hypertrophic growth involves cardiomyocyte enlargement rather than division, as adult cardiomyocytes have lost the ability to divide ( Porrello et al., 2013). Cardiac hypertrophy is an adaptive process which develops in response to physiological but also pathological processes, leading to heart muscle and cell hypertrophic growth with increased rigidity of the heart structures, and impaired diastolic function leading to heart failure with preserved ejection fraction (HFpEF) ( Loonat et al., 2019 Zhao et al., 2020). This development combined with the relative paucity of direct treatment options for cardiac hypertrophy makes continued research and the identification of novel therapeutic target molecules absolutely vital. Due to widespread risk factors such as obesity and smoking, the prevalence of hypertension and subsequent myocardial hypertrophy is rising, which poses a significant public health burden in an aging population ( Benjamin et al., 2018). Left ventricular hypertrophy is a consequence of hypertension in up to 30% of patients ( Cramariuc and Gerdts, 2016).

In vitro modeling serves as a vital tool for this further pathway analysis and treatment testing and has vastly improved over the recent years, providing a less costly and labor-intensive alternative to in vivo animal models. More recently discovered pathways showed the involvement of several non-coding RNAs, including micro RNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), which either promote or inhibit the remodeling process and pose a possible target for novel therapy approaches. Although several important pathways involved in the remodeling and hypertrophy process have been identified, further research is needed to achieve a better understanding and explore new and better treatment options.
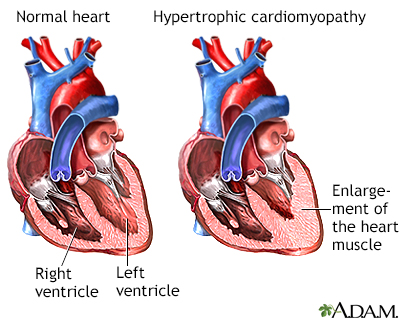
Pathological hypertrophy is a maladaptive response to stress conditions, such as pressure overload, and involve a number of changes in cellular mechanisms, gene expression and pathway regulations. Department of Cardiology, Medical University of Vienna, Vienna, AustriaĬardiac hypertrophy is an ongoing clinical challenge, as risk factors such as obesity, smoking and increasing age become more widespread, which lead to an increasing prevalence of developing hypertrophy.Nina Kastner †, Katrin Zlabinger †, Andreas Spannbauer, Denise Traxler, Julia Mester-Tonczar, Ena Hašimbegović and Mariann Gyöngyösi *


 0 kommentar(er)
0 kommentar(er)
